Density of Nacl Solution
The objective of this lab is to determine the average density of sodium chloride. No data available.
Density of some sugars alcohols.
. 5844 gmol Solubility. Iceliquid solution for T eq 001. If the sample body has mass m and it occupies volume V then the density of the substance from which it is composed can be calculated using the following formula.
71376 71386 Sodium chloride Halite Common Salt or Table Salt Rock Salt CAS number. Table 2 lists the simulation parameters for. Jonathan Pilafas performed on 9102018 Purpose.
- At standard room temperature. Melting Point MP sodium. No data available Specific gravity density.
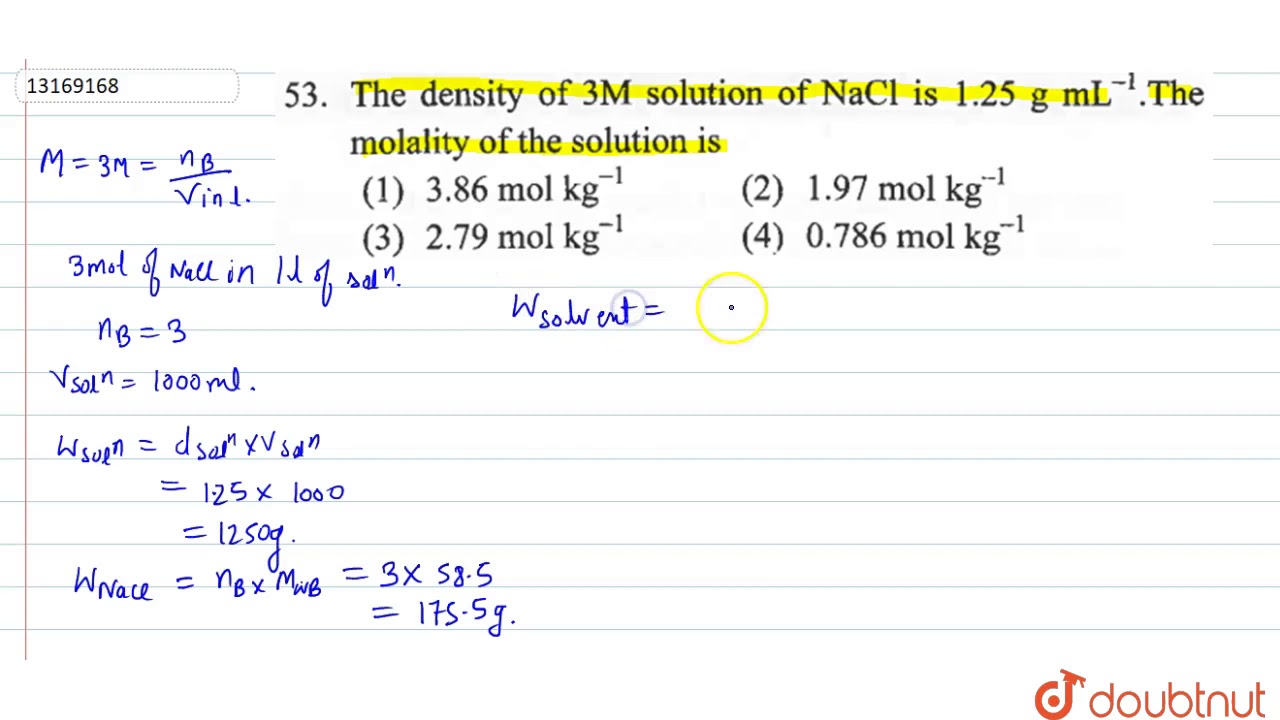
A linear relationship permits a reliable standard curve to be constructed see. 1033 gml Molecular mass. The density of 3M solution of NaCl is 125 gmL.
Calculate molality of the solution. The density of a saturated solution at 25C is 1202 gml. D m V.
The densities of saturated solutions of NaCl and KCL from 10 degrees to 105 degrees C. Sodium Chloride 5 wv. ρ is density n is refractive index at 589 nm clarification needed and η is viscosity all at 20 C.
Density of Aqueous Solutions of Organic Substances as Sugars and Alcohols - Changes in density of aqueous solutions with changes in concentration at 20C. Sodium chloride solution 5 M. Sodium chloride ˌsoʊdiəm ˈklɔːraɪd 8 commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl.
T eq is the equilibrium temperature between two phases. The table below gives the density kgL and the. What is the density of NaCl solution at 5 10 15 20 and 25.
According to the paper 27 the electrolyte density and dynamic viscosity were determined as 1138 10 3 kg m 3 and 913 mPa s respectively. Concentration of NaCl Solutions. Input a temperature and density within the range of the table to calculate for concentration or input concentration to calculate for density.
You will be following the procedure you used to find the density of pure water. Weigh out 333 g of salt and transfer it into a 100ml. In Imperial or US customary measurement system the density is equal to 135469 pound per cubic foot lbft³ or 1254 ounce per cubic inch ozinch³.
D dfrac m V. Preparations and Measurements of 100 NaCl Solution 1. Solution 3 Molar solution means there are 3 moles of Na Cl salt in 1 Liter.
Molecular weight of NaCl 5844. Hence there are 35844 gms in 1 Litre of water. Find Sigma-Aldrich-S6546 MSDS related peer-reviewed papers technical documents similar products more at.
Density volumemass Mass of 1 litre of solution 125 gmsml 1000 ml 1 250 gms V volume of. Molecular weight of Na Cl. The density of aqueous NaCl solutions is a nearly-linear function of the NaCl concentration in mass percent.
The Density Of 3m Solution Of Nacl Is 1 25 G Ml 1 The Molality Of The Solution Is Youtube

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
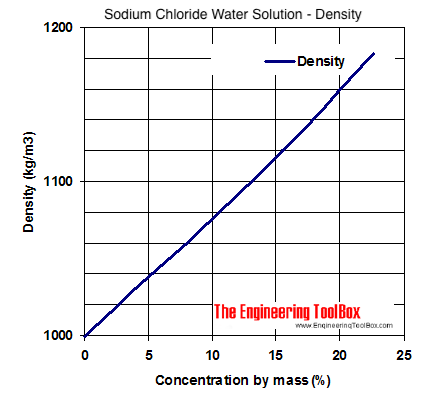
Sodium Chloride Water Solutions
0 Response to "Density of Nacl Solution"
Post a Comment